EASY
NEET
IMPORTANT
Earn 100
The density of acetic acid vapor at and is . The number of acetic acid molecules in the cluster that is formed in the gas phase is the closest to
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on States of Matter: Gases and Liquids
MEDIUM
NEET
IMPORTANT
The volume vs. temperature graph of mole of an ideal gas is given below. The pressure of the gas (in ) at and , respectively, are

EASY
NEET
IMPORTANT
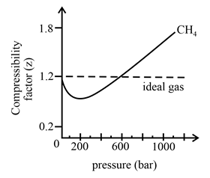
In the following compressibility factor vs. pressure graph at , the compressibility of at pressures deviates from ideal behaviour because:
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
If of water is introduced into a flask at , how many moles of water are in the vapour phase when equilibrium is established? (Given: Vapour pressure of at is )
HARD
NEET
IMPORTANT
Assertion: Van der Waals equation describes the behaviour of real gases.
Reason: The kinetic theory postulates that negligible volume of gaseous molecules and intermolecular forces of attraction do not stand correct at high pressure and low temperature.
